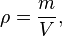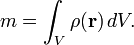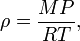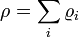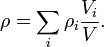Density
About this schools Wikipedia selection
SOS Children have produced a selection of wikipedia articles for schools since 2005. Sponsor a child to make a real difference.
The mass density or density of a material is its mass per unit volume. The symbol most often used for density is ρ (the lower case Greek letter rho). Mathematically, density is defined as mass divided by volume:
where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is also defined as its weight per unit volume, although this quantity is more properly called specific weight.
Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure but certain chemical compounds may be denser.
Less dense fluids float on more dense fluids if they do not mix. This concept can be extended, with some care, to less dense solids floating on more dense fluids. If the average density (including any air below the waterline) of an object is less than water it will float in water and if it is more than water it will sink in water.
Density is sometimes expressed by the dimensionless quantity " specific gravity" or " relative density", ie the ratio of the density of the material to that of a standard material, usually water. Thus a specific gravity less than one means that the substance floats in water.
The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Increasing the pressure on an object decreases the volume of the object and thus increases its density. Increasing the temperature of a substance (with a few exceptions) decreases its density by increasing its volume. In most materials, heating the bottom of a fluid results in convection of the heat from the bottom to the top, due to the decrease in the density of the heated fluid. This causes it to rise relative to more dense unheated material.
The reciprocal of the density of a substance is occasionally called its specific volume, a term sometimes used in thermodynamics. Density is an intensive property in that increasing the amount of a substance does not increase its density; rather it increases its mass.
History
In a well-known but probably apocryphal tale, Archimedes was given the task of determining whether King Hiero's goldsmith was embezzling gold during the manufacture of a golden wreath dedicated to the gods and replacing it with another, cheaper alloy. Archimedes knew that the irregularly shaped wreath could be crushed into a cube whose volume could be calculated easily and compared with the mass; but the king did not approve of this. Baffled, Archimedes is said to have taken an immersion bath and observed from the rise of the water upon entering that he could calculate the volume of the gold wreath through the displacement of the water. Upon this discovery, he leapt from his bath and ran naked through the streets shouting, "Eureka! Eureka!" (Εύρηκα! Greek "I have found it"). As a result, the term " eureka" entered common parlance and is used today to indicate a moment of enlightenment.
The story first appeared in written form in Vitruvius' books of architecture, two centuries after it supposedly took place. Some scholars have doubted the accuracy of this tale, saying among other things that the method would have required precise measurements that would have been difficult to make at the time.
From the equation for density (ρ = m / V), mass density has units of mass divided by volume. As there are many units of mass and volume covering many different magnitudes there are a large number of units for mass density in use. The SI unit of kilogram per cubic metre (kg/m3) and the cgs unit of gram per cubic centimetre (g/cm3) are probably the most commonly used units for density. (The cubic centimeter can be alternately called a millilitre or a cc.) 1,000 kg/m3 equals one g/cm3. In industry, other larger or smaller units of mass and or volume are often more practical and US customary units may be used. See below for a list of some of the most common units of density.
Measurement of density
The density at all points of a homogeneous object equals its total mass divided by its total volume. The mass is normally measured with a scale or balance; the volume may be measured directly (from the geometry of the object) or by the displacement of a fluid. To determine the density of a liquid or a gas, a hydrometer or dasymeter may be used, respectively. Similarly, hydrostatic weighing uses the displacement of water due to a submerged object to determine the density of the object.
If the body is not homogeneous, then its density varies between different regions of the object. In that case the density around any given location is determined by calculating the density of a small volume around that location. In the limit of an infinitesimal volume the density of an inhomogeneous object at a point becomes: ρ(r) = dm/dV, where dV is an elementary volume at position r. The mass of the body then can be expressed as
The density of granular material can be ambiguous, depending on exactly how its volume is defined, and this may cause confusion in measurement. A common example is sand: if it is gently poured into a container, the density will be low; if the same sand is then compacted, it will occupy less volume and consequently exhibit a greater density. This is because sand, like all powders and granular solids, contains a lot of air space in between individual grains. The density of the material including the air spaces is the bulk density, which differs significantly from the density of an individual grain of sand with no air included.
Changes of density
In general, density can be changed by changing either the pressure or the temperature. Increasing the pressure always increases the density of a material. Increasing the temperature generally decreases the density, but there are notable exceptions to this generalization. For example, the density of water increases between its melting point at 0 °C and 4 °C; similar behaviour is observed in silicon at low temperatures.
The effect of pressure and temperature on the densities of liquids and solids is small. The compressibility for a typical liquid or solid is 10−6 bar−1 (1 bar = 0.1 MPa) and a typical thermal expansivity is 10−5 K−1. This roughly translates into needing around ten thousand times atmospheric pressure to reduce the volume of a substance by one percent. (Although the pressures needed may be around a thousand times smaller for sandy soil and some clays.) A one percent expansion of volume typically requires a temperature increase on the order of thousands of degrees Celsius.
In contrast, the density of gases is strongly affected by pressure. The density of an ideal gas is
where M is the molar mass, P is the pressure, R is the universal gas constant, and T is the absolute temperature. This means that the density of an ideal gas can be doubled by doubling the pressure, or by halving the absolute temperature.
In the case of volumic thermal expansion at constant pressure and small intervals of temperature the temperature dependence of density is :
where 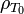 is the density at a reference temperature,
is the density at a reference temperature,  is the thermal expansion coefficient of the material at temperatures close to
is the thermal expansion coefficient of the material at temperatures close to 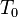 .
.
Density of solutions
The density of a solution is the sum of mass (massic) concentrations of the components of that solution.
Mass (massic) concentration of each given component ρi in a solution sums to density of the solution.
Expressed as a function of the densities of pure components of the mixture and their volume participation, it reads:
provided that there is no interaction between the components.
Densities
Water
Density of water at 1 atm pressure:
| Temp (°C) | Density (kg/m3) |
|---|---|
| 100 | 958.4 |
| 80 | 971.8 |
| 60 | 983.2 |
| 40 | 992.2 |
| 30 | 995.6502 |
| 25 | 997.0479 |
| 22 | 997.7735 |
| 20 | 998.2071 |
| 15 | 999.1026 |
| 10 | 999.7026 |
| 4 | 999.9720 |
| 0 | 999.8395 |
| −10 | 998.117 |
| −20 | 993.547 |
| −30 | 983.854 |
| The values below 0 °C refer to supercooled water. | |
Air
Density of air at 1 atm pressure:
| T (°C) | ρ (kg/m3) |
|---|---|
| −25 | 1.423 |
| −20 | 1.395 |
| −15 | 1.368 |
| −10 | 1.342 |
| −5 | 1.316 |
| 0 | 1.293 |
| 5 | 1.269 |
| 10 | 1.247 |
| 15 | 1.225 |
| 20 | 1.204 |
| 25 | 1.184 |
| 30 | 1.164 |
| 35 | 1.146 |
Various materials
Unless otherwise noted, all densities given are at standard conditions for temperature and pressure, that is, 273.15 K (0.00 °C) and 100 kPa (0.987 atm).
| Material | ρ (kg/m3) | Notes |
|---|---|---|
| Air | 1.2 | At sea level |
| Aerographite | 0.2 | * |
| Metallic microlattice | 0.9 | * |
| Aerogel | 1.0 | * |
| Styrofoam | 75 | Approx. |
| liquid hydrogen | 70 | At ~ -255°C |
| Cork | 240 | Approx. |
| Lithium | 535 | |
| Wood | 700 | Seasoned, typical |
| Potassium | 860 | |
| Sodium | 970 | |
| Ice | 916.7 | At temperature < 0°C |
| Water (fresh) | 1,000 | |
| Water (salt) | 1,030 | |
| Plastics | 1,175 | Approx.; for polypropylene and PETE/ PVC |
| Tetrachloroethene | 1,622 | |
| Magnesium | 1,740 | |
| Beryllium | 1,850 | |
| Glycerol | 1,261 | |
| Silicon | 2,330 | |
| Aluminium | 2,700 | |
| Diiodomethane | 3,325 | liquid at room temperature |
| Diamond | 3,500 | |
| Titanium | 4,540 | |
| Selenium | 4,800 | |
| Vanadium | 6,100 | |
| Antimony | 6,690 | |
| Zinc | 7,000 | |
| Chromium | 7,200 | |
| Manganese | 7,325 | Approx. |
| Tin | 7,310 | |
| Iron | 7,870 | |
| Niobium | 8,570 | |
| Cadmium | 8,650 | |
| Cobalt | 8,900 | |
| Nickel | 8,900 | |
| Copper | 8,940 | |
| Bismuth | 9,750 | |
| Molybdenum | 10,220 | |
| Silver | 10,500 | |
| Lead | 11,340 | |
| Thorium | 11,700 | |
| Rhodium | 12,410 | |
| Mercury | 13,546 | |
| Tantalum | 16,600 | |
| Uranium | 18,800 | |
| Tungsten | 19,300 | |
| Gold | 19,320 | |
| Plutonium | 19,840 | |
| Platinum | 21,450 | |
| Iridium | 22,420 | |
| Osmium | 22,570 |
*Air excluded when calculating density
Others
| Entity | ρ (kg/m3) | Notes |
|---|---|---|
| Interstellar medium | 1×10−19 | Assuming 90% H, 10% He; variable T |
| The Earth | 5,515 | Mean density. |
| The Inner Core of the Earth | 13,000 | Approx., as listed in Earth. |
| The core of the Sun | 33,000–160,000 | Approx. |
| Super-massive Black hole | 9×105 | Density of a 4.5-million-solar-mass black hole Event horizon radius is 13.5 million km. |
| White dwarf star | 2.1×109 | Approx. |
| Atomic nuclei | 2.3×1017 | Does not depend strongly on size of nucleus |
| Neutron star | 1×1018 | |
| Stellar-Mass Black hole | 1×1018 | Density of a 4-solar-mass black hole Event horizon radius is 12 km. |
Other common units
The SI unit for density is:
- kilograms per cubic meter (kg/m3)
Litres and metric tons are not part of the SI, but are acceptable for use with it, leading to the following units:
- kilograms per liter (kg/L)
- grams per milliliter (g/mL)
- metric tons per cubic meter (t/m3)
Densities using the following metric units all have exactly the same numerical value, one thousandth of the value in (kg/m3). Liquid water has a density of about 1 kg/dm3, making any of these SI units numerically convenient to use as most solids and liquids have densities between 0.1 and 20 kg/dm3.
- kilograms per cubic decimetre (kg/dm3)
- grams per cubic centimetre (g/cc, gm/cc or g/cm3)
- 1 gram/cm3 = 1000 kg/m3
- megagrams (metric tons) per cubic metre (Mg/m3)
In US customary units density can be stated in:
- Avoirdupois ounces per cubic inch (oz/cu in)
- Avoirdupois pounds per cubic inch (lb/cu in)
- pounds per cubic foot (lb/cu ft)
- pounds per cubic yard (lb/cu yd)
- pounds per US liquid gallon (lb/gal)
- pounds per US bushel (lb/bu)
- slugs per cubic foot
Imperial units differing from the above (as the Imperial gallon and bushel differ from the US units) in practice are rarely used, though found in older documents. The density of precious metals could conceivably be based on Troy ounces and pounds, a possible cause of confusion.